
How to Draw the Lewis Dot Structure for BeCl2 Beryllium chloride YouTube
BeCl2 Lewis structure contains beryllium atom in central position whereas both chlorine atom on either side of it. There is no lone pair present on the central atom but 3 lone pairs present on each outer atom in the lewis dot structure of BeCl2.

What is the structure of becl2 molecule in gaseous and solid state
A step-by-step explanation of how to draw the BeCl2 Lewis Dot Structure (Beryllium chloride).For the BeCl2 structure use the periodic table to find the total.

Is BeCl2 Polar or Nonpolar? Techiescientist
The beryllium atom in a gaseous BeCl2 molecule is an example of a central atom with no lone pairs of electrons in a linear arrangement of three atoms.. (90°). Sulfur is in the same group as oxygen, and H 2 S has a similar Lewis structure. However, it has a much smaller bond angle (92.1°), which indicates much less hybridization on sulfur.
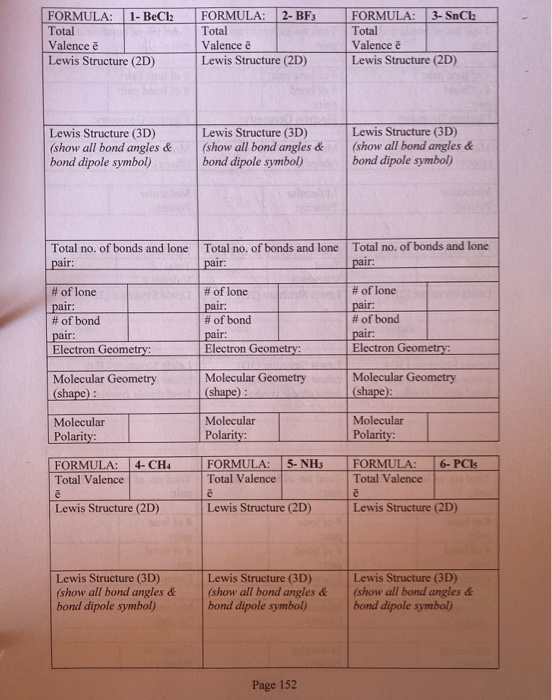
Solved FORMULA 1 BeCl2 Total Valence Lewis Structure (2D)
Electron Deficient Species. Good examples of the first type of exception are provided by BeCl 2 and BCl 3.Beryllium dichloride, BeCl 2, is a covalent rather than an ionic substance.Solid BeCl 2 has a relatively Complex structure at room temperature, but when it is heated to 750°C, a vapor which consists of separate BeCl 2 molecules is obtained. Since Cl atoms do not readily form multiple.
Fcl2 Lewis Structure What is the molecular shape of BeCl2? YouTube
Lewis structure of BeCl2 contains two single bonds between the Beryllium (Be) atom and each Chlorine (Cl) atom. The Beryllium atom (Be) is at the center and it is surrounded by 2 Chlorine atoms (Cl). The Beryllium does not have lone pairs while both the Chlorine atoms have 3 lone pairs.
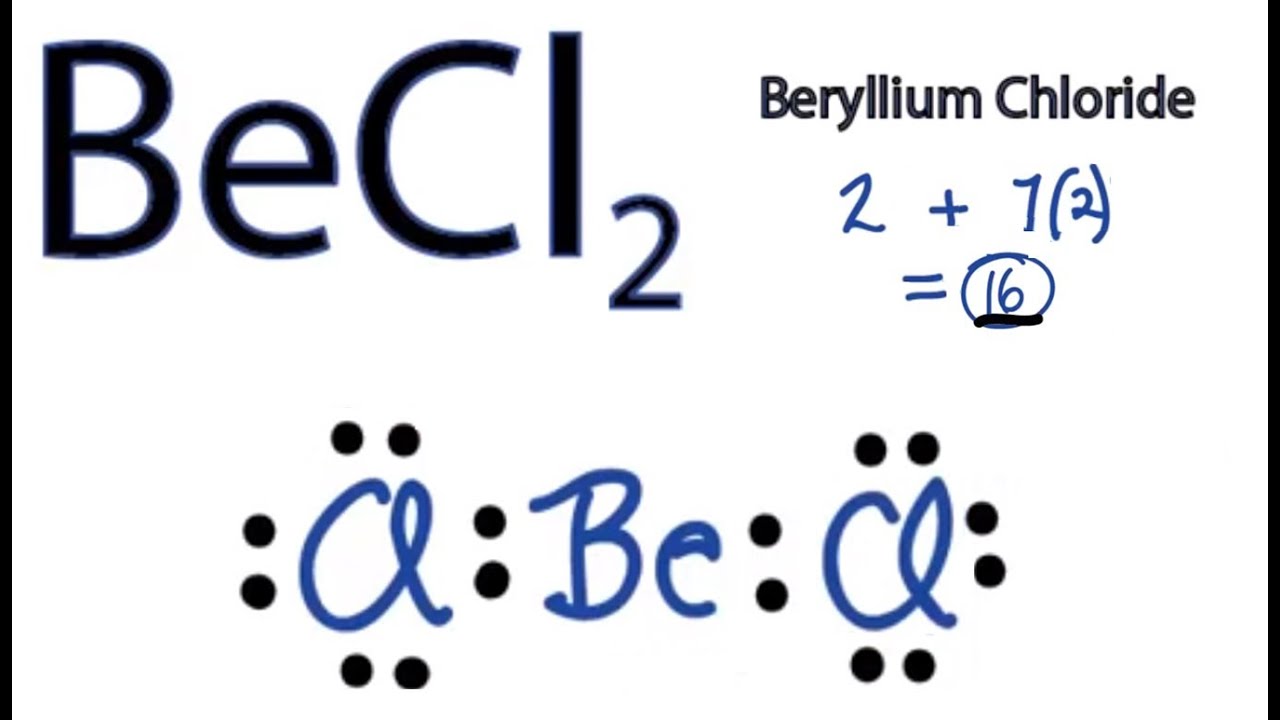
BeCl2 Lewis Structure How to Draw the Lewis Structure for BeCl2 YouTube
BeCl2 is a molecular (covalent) compound in the gas phase. It is a linear molecule and beryllium is an exception to the octet rule.In the solid phase, you ca.

BeCl2 Molecular Geometry Science Education and Tutorials
Subscribed 24K views 3 years ago An explanation of the molecular geometry for the BeCl2 (Beryllium chloride) including a description of the BeCl2 bond angles. The electron geometry for the.

BeCl2 Lewis Structure (Beryllium Chloride) YouTube
BeCl2 Lewis Structure - How to Draw the Lewis Structure for BeCl2 Wayne Breslyn 721K subscribers Join Subscribe Subscribed 1.1K 161K views 10 years ago A step-by-step explanation of how to.

Becl2 Electron Pair Geometry
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

How to draw BeCl2 Lewis Structure? Science Education and Tutorials
January 3, 2024 Techiescientist Science is fun! Home Advertise with us About Me Privacy Policy Search for: Main Menu BeCl2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram BeCl2 referred to as Beryllium Chloride, is an inorganic compound. It appears as white or yellow crystal solid at room temperature.

BeCl2 Lewis Structure & VSEPR Geometry YouTube
The BeCl 2 Lewis structure is similar to BeF 2 since F is in Group 7 and has 7 valence electrons. Beryllium (Be) doesn't need 8 valence electrons to have an octet (Be often only needs 4). If you're not sure you have the best Lewis structure for BeCl 2 you can calculate the formal charges. You'll find the Be in BeCl 2 only has 4 valence electrons.
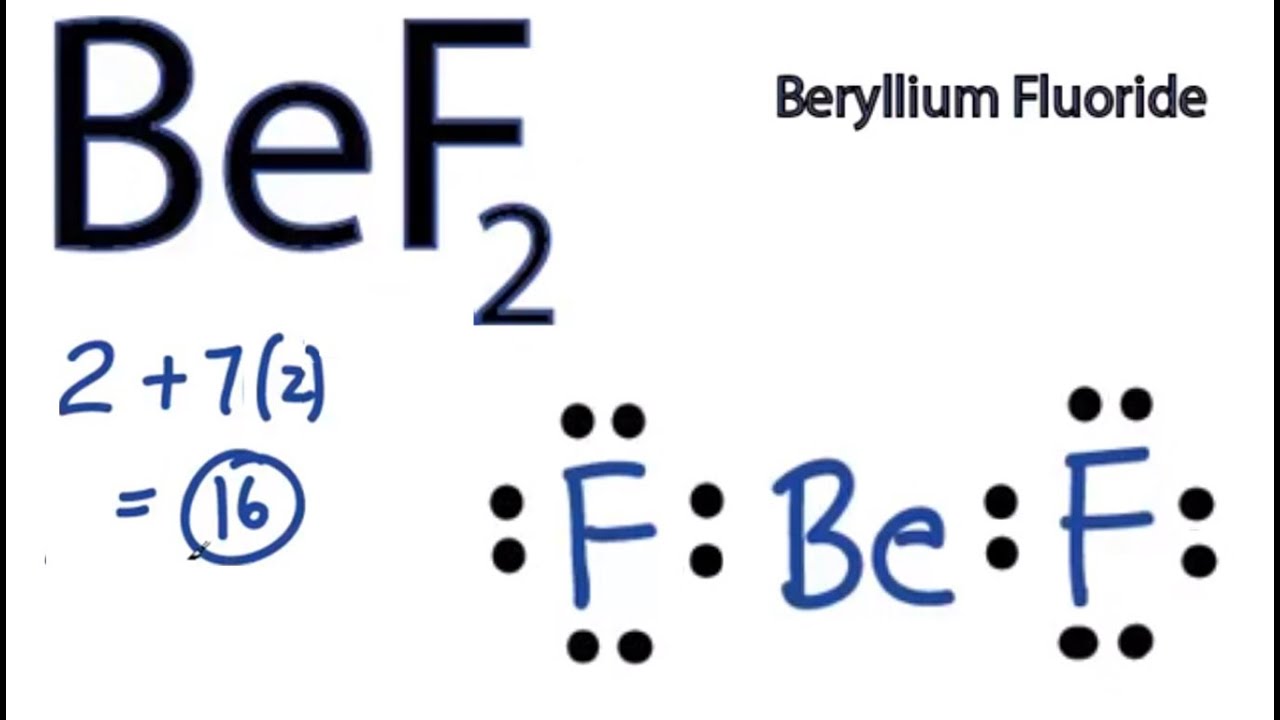
25 Bohr Diagram For Beryllium Wiring Database 2020
= 16 Thus, BeCl2 has 16 valence electrons. BeCl2 Lewis Structure The Lewis structure of any given molecule helps to know the arrangement of atoms in the molecule, bond formations and the lone pairs. All these characteristics help in determining other properties of the molecule.
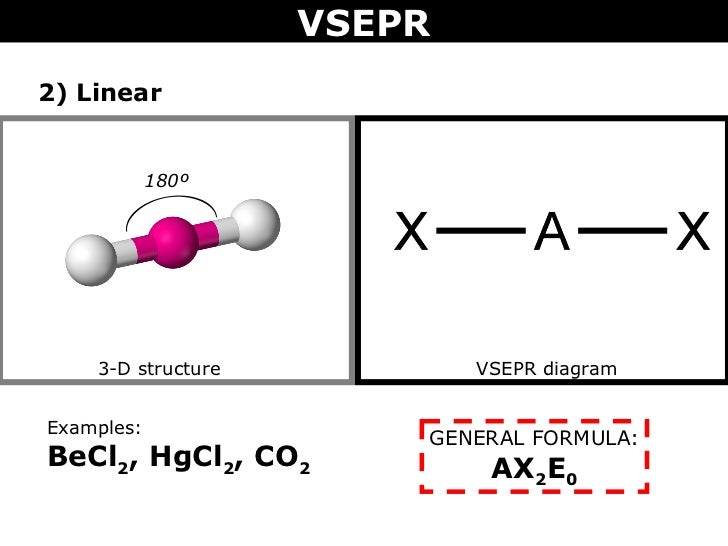
BeCl_2 and TeCl_2 are both covalent molecules, yet BeCl_2 is linear
BeCl2 lewis structure has a Beryllium atom (Be) at the center which is surrounded by two Chlorine atoms (Cl). There are 2 single bonds between the Beryllium atom (Be) and each Chlorine atom (Cl). There are 3 lone pairs on both the Chlorine atoms (Cl).
[Solved] 10. (5 pts.) BeCl2 LEWIS STRUCTURE WITH ANY RESONANCE
.more It's cable reimagined No DVR space limits. No long-term contract. No hidden fees. No cable box. No problems. Hey Guys,In this video, we are going to learn about the Lewis structure of.

How to draw BeCl2 Lewis Structure? Science Education and Tutorials
Beryllium chloride has a significant ionic contribution to its electronic structure. A quick calculation, DF-BP86/def2-SVP, reveals that the charge of beryllium is q(Be) = 0.8 q ( B e) = 0.8 and of chlorine q(Cl) = −0.4 q ( C l) = − 0.4, based on natural bond orbital analysis. The Lewis structures best describing this are depicted below.

BeCl2 Lewis structure, Molecular geometry, Hybridization, Bond angle
A three-step approach for drawing the BeCl2 Lewis structure can be used. The first step is to sketch the Lewis structure of the BeCl2 molecule, to add valence electron around the Beryllium atom; the second step is to valence electron to the two chlorine atoms, and the final step is to combine the step1 and step2 to get the BeCl2 Lewis Structure.